RT-PCR
Most Common Technique for seeing molecular markers|
(RNA extraction with RNA-STAT) 1. Mix six embryos with 800 µl of RNA-Stat. Note. Amount of Total RNA are 3-4 µg/embryo and 300-400 ng/AC. 2. Add 200 µl of choloroform and recover the supernatant (100 µl, mix very well). 3. Centrifuge at maximum speed (e.g. 15k rpm) for 10 min at 4 ºC. 4. Recover the supernatant (usually 400 µl) and do a chloroform extraction. 5. Save 380 µl of supernatant and add 380 µl of isopropanol. 6. Mixed well and incubate in dryice until freeze or in -80 ºC for 30 min. Note. You can store sample in -20 ºC forever. 7. Centrifuge at maximum speed for 15 min at 4 ºC. 8. Wash the pellet with 70% EtOH and dissolve pellet into 12 µl of water. Note. Use 5 µl for the following Northern Blot reaction. (Loading Sample)
If you have 8 samples---- 1. Mix 24 x 8.5 µl of Master Mix, 0.6 x 8.5 µl of RNA guard, and 1.5 x 8.5 µl of MMLV RT enzyme very well (Called as Master Enzyme Mix). 2. Mix 4 µl of RNA solution and 26 µl of Master Enzyme Mix very well. 3. Incubate at 42 ºC for 1 h. 4. Add 30 µl of water. 5. Store at -20 ºC (Use as soon as possible. Do not keep for more than 3 months). (PCR)
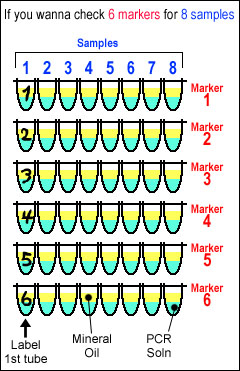 In the case that you have 8 samples and want to check 6 markers for each samples-------Please always refer to the letter color, above table, and right figure. In the case that you have 8 samples and want to check 6 markers for each samples-------Please always refer to the letter color, above table, and right figure.(Set up the following under the cold condition) 0-1. Set up PCR machine and prepare six 8-connected PCR tubes. 0-2. Put 3 µl of cDNA soln into PCR tube and keep them in 4 ºC. 0-3. Put 2 x 8.5 µl of primers into 1.5 ml tubes (total six tubes). RADIO ACTIVE START from HERE!!! BE CAREFUL!!! 1. Mix 15 x 9 x 6 µl of PCR Master Mix, 0.3 x 9 x 6 µl of Taq polymerase, and 0.1 x 9 x 6 µl of α-32P-dCTP (called as PCR Master Enzyme Soln). 2. Mix 15.4 x 8.5 µl of PCR Master Enzyme Soln into 1.5 ml tubes with primers (see 0-3) very well (called as PCR Master Enzyme&Primer Soln). 3. Put 17.4 µl of PCR Master Enzyme&Primer Soln into each PCR tube (see 0-2). 4. Add one drop of mineral oil into each PCR tubes. 5. Close tubes and start PCR. 6. Run on a 5 % acrylamide gel after PCR.
|
Major markers:
ODC: Always good loading control marker EF1α: Much stronger than ODC but not good before gastrula. |